From the World, for the World
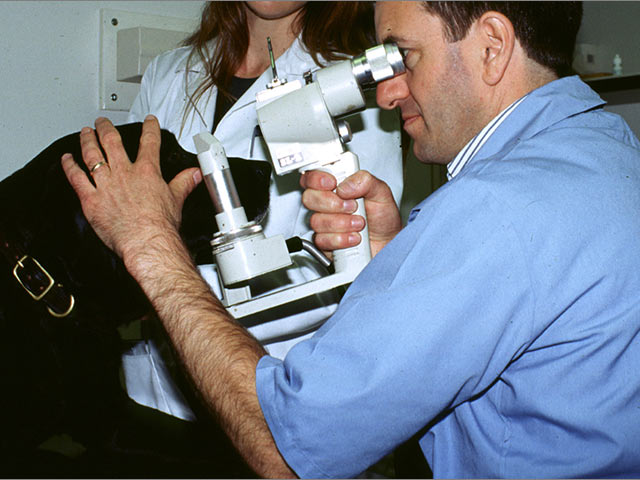



When researchers want to study the basic chemistry of canine diseases, they can put together various chemical compounds, or perhaps run tests on blood samples or specific types of skin samples donated from dog owners. The resuts scientists get from these investigations are valuable, yielding basic facts about cell function and interactions of various compounds. But what scientists cannot determine from this kind of research is how a disease or condition plays out in the real world, where different dogs, living in different environments with different owners, eating different foods, living different lives, experience the same condition. To investigate this messier kind of science, researchers turn to clinical trials.
What Is a Clinical Trial?
Dr. David Maggs, associate professor of opthamology and board-certified opthamologist at the University of California at Davis, has done both clinical trial research as well as more basic research, or what he and other refer to as "benchtop research." He emphasizes that clinical trials are still a form of research, with the same rigorous scienctific thinking. The differences are in what scientists are looking for when they do clinical trials. "When we grow cells in a petri dish, that's very easy to control," he says. "We can get some very exacting answers doing that kind of research. But who knows if what we see extrapolates into the whole animal?"
Dr. Maggs says there are a couple of different ways to get this kind of extrapolation. "We could use laboratory animals, ones that are not owned by anyone. The advantage here is that we gain a lot more control over variables like age, nutriution, gender. The disadvantage is...we have to use live animals."
Illustrating the use of clinical trials, Dr. Maggs describes a current effort he is undertaking to treat a condition known as "indolent ulcers," an uncomfortable growth that forms in the eyes of Boxers and other breeds. These ulcers resist standard therapies for treating eye ailments. This prompted Dr. Maggs to search for something new to relieve the dogs' suffering. He decided that the most rigorous way to get an answer was to recruit owners with dogs that suffer from this condition, ask them to come to his lab and try a new method that is still unproven. This method involves making a medication out of the dog's own blood serum and applying it directly to the ulcer. The indolent ulcer trial is still underway, but the results so far are encouraging. The steps to develop the study and get results exemplify many factors common to all clinical trials.
To test the effectiveness of the serum therapy, Dr. Maggs enrolls dogs for a four-week period. During that time, the owners are asked to administer the serum and report on progress. To assure that bias is removed from the study, a double-blind design is set up for the work. "Half the dogs receive the serum and half the dogs will receive some other, standard form of treatment. Neither the owners nor the researchers know which dogs are getting which treatment."
Dr. Jeffrey Klausner is the president and CEO of the Animal Medical Center of New York City, a position he has had for six months. After 33 years on the faculty of the University of Minnesota Veterinary College, Dr. Klausner has seen numerous clinical trials. A current trial underway at the AMC involves the use of a new type of vaccine. The first stage of the study went very well, and they are now expanding the recruitment effort to include a larger number of dogs in a second, proving phase of the trial. Dr. Klausner says that in all clinical trial work, the need to remove bias is a crucial element in designing an effective trial that veterinarians and dog owners will trust. "If researchers think that something is a miraculous new treatment or know what they are looking for, that might skew the results. The same happens with owners. It's very important for us to make sure that no one knows which therapies any of the dogs are getting." Dr. Klausner says that if all goes well with this second phase of the trial, the vaccine coule become standard protocol for veterinary clinics.
Even the most serious diseases can be better understood through clinical trials. Dr. Susan Lana, associate professor of oncology at Colorado State University, is currently involved in a clinical trial examining forms of canine cancer that have not responded to other treatments. "The standard treatment for tumors is surgery and amputation," she explains. "But for many owners that is just not an option. We are investigating new radiation therapies, completely novel chemotherapies, or some new combination of radiation and chemotherapy. We are also looking at agents that we can use to target particular kinds of tumors."
With the patient living in the real world, like a normal dog, periodically coming to the research facility, researchers can gain from trials vastly more information than any laboratory-only effort. As Dr. Lana explains,"We can constantly measure efficacy. If the client is responding to the treatment, then drops for some reason, we can increase the dose. Also, we can figure out which kinds of tumors the treatment works on. Through the trial, we are finding the right therapy, the right dose, and learning more about the tumors. We can also learn which how drugs work in combination in the dog's body, how the body metabolizes different drugs as you add new ones on."
Recruiting and Enrolling Participants
Because clinical trials rely on dog owners to get involved, there is almost always some kind of recruitment effort as part of the study. At the University of California at Davis, Terri Geuerro, clinical trials coordinator, is responsible for helping to build a base of participants, as well as following up on other stages after a trial is underway. "I'll hear from a drug company that they want to test the effectiveness of a new treatment. We post the information on our Web site, and when people come in with their dogs for a visit, I'll talk to them about standard treatments. Then I do a blood test, and a team of scientists tells the client what treatment options are available. If the client meets the eligibility requirements, we'll them about the trial."
There are strict rules in place about recruitment. Owners must be fully informed about every possible treatment, and why standard choices are not likely to be effective for their dog. The owner is then given extensive printed materials, outlining exactly what would happen, and what is expected of them, in the trial. "After we give them all that information," Ms. Guerrero explains, "we let the client decide which way to go."
The recruitment effort often includes financial compensation. Depending on the treatment being offered, this can make a big difference in people's ability to provide their dogs with effective new treatments. "We see people from all walks of life – some can drop $1,000 in a visit, others are really strapped. It's one of the main incentives for a lot of people to come out for clinical trials. They want to help their animals, but don't have the finances to do so. With support, they get to help their dogs and contribute to the science."
Dr. Lana says that being able to offer people financial help is one of her favorite parts of doing clinical trials. "Often, people will get access to an expensive surgical technique or procedure they could not afford. These are people who really want to treat their animals, and the clinical trials give them more options."
Worthwhile Challenges
Clinical trials allow researchers to get rigorous answers, without the ethical issues of using live animals. Other advantages are that researchers can obtain more solid proof of their proposed treatment. "You are looking at the real disease," Dr. Maggs explains. "If your drug or your test works with those dogs, no extrapolation is necessary because you're looking at the very population in which it will be used."
Of course, because the subjects are coming from the real world, the gains scientists see in the strength of their results is challenged by the fact that dogs from the real world are less controlled, less predictable. "There are more variables," Dr. Maggs explains. "You get dogs of every age, every breed, every gender, every body weight."
Another challenge, moving away from strictly laboratory-based investigations, is the introduction of humans into the study, dog owners who must be trusted to follow instructions on administering medications in ways that bring about reliable results. Those owners are rarely trained in the rigours of scientific methods, and their subjectivity can affect the progress of the research.
Dr. Maggs says that all the challenges of doing clinical trials is worth it for the reliability of the results. With clinical trials, both the researcher and the dog owner can have more confidence that the new therapy or treatment is effective.
Design and Ethical Oversight
With all the talk of financial incentives, private phramaceutical companies asking universities to run clinical trials on potential new medications, and trying out untested therapies, it might appear that clinical trials run the risk of treating dogs, and by extension their owners, like guinea pigs. This is far from the truth. Every aspect of clinical trials with dogs are vigorously regulated, from the design of the study, right down to the smallest bit of information that is shared with clients. Researchers who have an idea for a clinical trial must submit their concept to two review boards: one at their specific research facility, and another with national membership.
Once the researchers have an idea that they believe is in line with accepted principles, they will submit their clinical trial design to the oversight committees. It is not uncommon for the design to be revised by the oversight committee and returned to the researchers before the trial can begin. The material researchers must submit is quite specific. As Terri Guerrero explains, there is an organization known as the Institute of Animal Care Use Committee (IACUC). Under the IACUC system, every research organization must have an oversight board that consists of at least one researcher, a veterinarian, and a layperson who can comment on the concept from the point of view of a regular dog owner. The committee parses through each detail of the proposed trial Part of Ms. Guerrero's job is putting together all the materials requested by the UC Davis IACUC committee. "If we come up with a study and part of it involves taking 50 ML of blood from each dog," Guerrero says, "the IACUC committee would demand we do it a certain way, or else they'll say we can't do the study." For example, IACUC has regulations on how much blood per pound of body weight can be drawn from dogs in any study.
Ms. Guerrero says that the regulations for clinical trials in dogs rival the requirements in human trials. "A lot of people might not realize how much is done to protect every dog that is part of a study. Researchers have a lot of responsibilities, and when they design a study, they are not going to do anything unethical or dangerous to the dogs."
Dr. Lana, operating for many years under the strict guidelines of the Animal Care and Use Committee at Colorado State University, sums up the system this way: "There must be a belief that the goals of the trial are realistic and that we expect information and benefit from doing all this. There has to be a very good reason to try the things we want to test."
Putting it more bluntly, Dr. Maggs explains, "We're not allowed to go hog-wild on client-owned dogs! We have to have a basis from the lab and proof of principle that supports the idea that what we want to test has potential efficacy. It has to be humane, morally and ethically sound."
Part of the IACUC oversight includes communications with owners. Researchers may be very excited about a new therapy, and eager to sign up participants. But every one of those participants must know precisely what they are signing up for. Dr. Klausner puts it this way: "We only do clinical trials with the ful, knowledgeable consent of the owners. We want them to know what we're doing, because we are all in this for the same reason: we want to improve the health of the dogs."
The requirements go even further. Clinical trials are a form of research, and by their very nature study something that is new, and almost always untested. But each clinical trial must prove up front that nothing else is already available to treat this condition effectively. There must be a population of dogs that has no standard options. "If we know something already works," Dr. Klausner explains, "we would not run a clinical trial with an untested procedure."
Even more specific, when a novel idea is approved for a clinical trial, the researchers still have to show, on a client-by-client basis, that an existing therapy will not cure the dog or improve the dog's condition. "Part of being humane," Dr. Maggs says, "is that we would not withhold something that is known and proven to work better. In my field, it would be completely unacceptable to withhold a gold standard treatment for dry eye just because I wanted to dabble in something new."
All of this oversight may seem daunting. But all researchers agree that knowing such regulations exist keeps them constantly questioning the basics of what they are trying to prove and discover. It also raises the entire field of clinical trials to a higher level, making it more likely that dog owners will freely participate.
Putting the Results to Good Use
Once a clinical trial has been initiated, it can run for any length of time, depending on the condition researchers are focusing on. Just as the subjects of the clinical trials come from the real world, the results are meant to go back into the real world in the form of practical veterinary techniques or new medications that can be repeated countlessly, for dogs anywhere in the world.
How do the positive results of an opthamology trial in Davis, California, make it to a rural veterinary clinic in the northern woods of Maine, or to a small town in Arkansas, so clients whose dogs have indolent ulcers can be treated successfully? One way is through publication in veterinary journals, where the researchers who did the study detail their efforts and explain the benefits. Dr. Maggs has often written about successful clincal trials in journals, and says that when veterinarians begin using a published technique with their clients, that creates a sort of extended test, further proving the value of the idea. "That's the best of all – if we can get our veterinary colleagues to re-rest it for themselves and see if they get the same results. That proves whether it works in the real world. If they support our findings, that's great. If they don't, we still want to hear from them in case there is some data we might have missed."
Dr. Klausner says that, right from the start, when his organization gets involved in clinical trials he and his researchers envision the way their work will be used. "When people walk into their veterinarian's office, they want to hear about the most effective therapies. Clinical trials are one very important way that the benefit of a drug or some other substance can be made available to them. If you don't have trials, then all you really have is suggestions that something might work without any real proof."
University research facilities do not have anywhere near as much access to clients as veterinary clinics. In order to recruit the many dogs needed for a reliable trial, which can often number 100 or more, veterinary colleges and universities will form partnerships with private veterinary clinics for the trials. Dr. Patricia Smith, D.V.M., Ph.D., a veterinary opthamologist at a the Animal Eye Care clinic in Fremont, CA, has formed a partnership with Dr. Maggs and his staff at UC Davis to help recruit dogs for the indolent ulcer trial. "We see a large volume of cases," Dr. Smith explains. "To get statistics, they might need 100 cases. It would take the university several years to see that many cases, but with a private opthamologist helping out, it might only take a couple of years."
Dr. Smith follows the same ethical guidelines demanded of researchers. She must make sure that the dog has not responded to other, standard treatments. But when a dog is suffering from an indolent ulcer and has not yet found a cure, she is proud to offer the idea of a clinical trial. "I can tell owners that there is a trial where they can get brand-name medications for free that might heal their dog. The clients benefit financially, and at the same time they are helping to eventually prove whether the treatment could help all dogs with the same condition."
Encouraging More Participation
Researchers across the country are actively designing new clinical trials in almost every canine condition you can name, from those that are, at worst, simply irritating like skin condition, to the most threatening cancers. In every case, they need dogs to participate, owners who are willing to work with researchers to test and prove new therapies with potential. After the initial design and approval phase, getting enough participants for a study is the primary endeavor of the researchers. When owners see a condition appear in their dogs, especially pure breeds, they are encouraged to go on the Web and see which veterinary colleges and universities in their area are running clinical trials, or ask veterinarians if they know of any trials for the condition.
As Dr. Klausner emphasizes, "There is no National Institute of Health for dogs. So owners and breeders need to do it. They need to bring their dogs to clinical trials whenever those dogs have a disease or a predisposition to a disease. We need numbers, partners providing cases. That's what owners and breeders can do to eliminate diseases from their breeds."
Terri Guerrero explains that there is something for every breeder, across the pure-bred spectrum. "There are people out there who want to know why we see more lymphomas in Golden Retrievers or why there are more mass-cell tumors in Australian Shepherds. They can help us collect the information through clinical trials so we can learn new ways to prevent these diseases."
All dog owners want to know that treatments they get from their veterinarians are reliable and effective. Clinical trials give them a way to help make that happen. Of course, that means the owners get a taste of the rigorous oversight researchers must follow. "When people do clinical trials," Dr. Maggs explains, "they should understand that they are becoming researchers themselves and they are getting involved in the research project. The way owners perform during the trials may determine treatments for other dogs far into the future. They should stay in close touch with the researchers throughout the study and not try to manipulate doses or anything on their own."
Dr. Maggs says that it all comes down to trust in people to participate fully and intelligently. It may be a risk, but it makes perfect sense: to apply to the real world, the test must be done in the real world. "Owners are taking on a big responsibiloity to help assure that the results give us safe procedures for the next generation of dogs."
